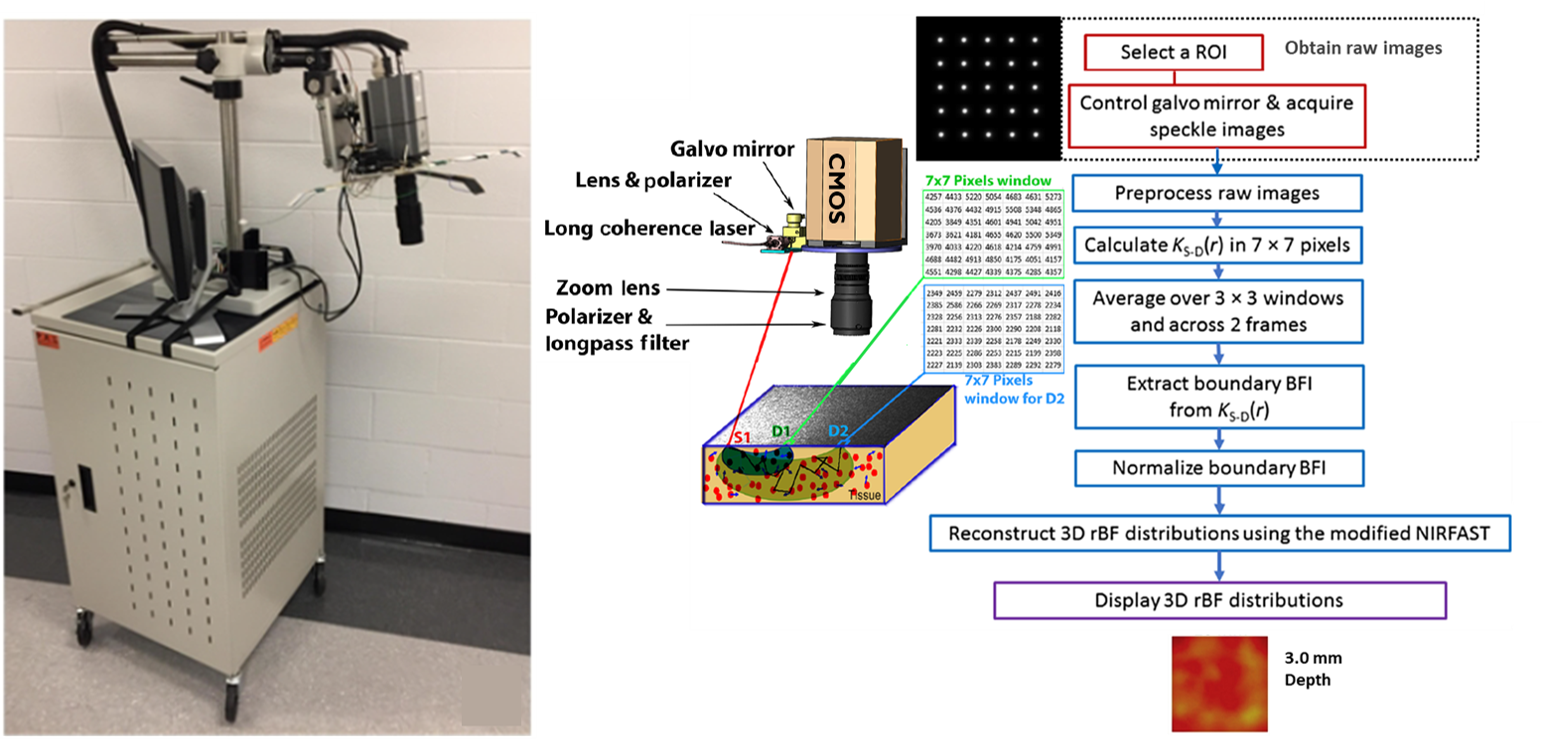
Speckle contrast diffuse correlation tomography (scDCT; US Patent #9861319, 2016-2036). This study combines the benefits of fast and high-resolution CCD detection with the unique finite-element-method (FEM)-based DCT reconstruction technique allowing for 3-D imaging of blood flow distributions in relatively deep tissues (up to ∼10 mm). What distinguishes the CCD detection in scDCT from the APD detection in DCS/DCT is the transition from measurements of slower temporal fluctuations (hundreds of milliseconds) to faster spatial fluctuations (a few milliseconds) of laser speckles, allowing for much higher temporal resolution. Furthermore, hundreds of 2-D-array detectors provided by the CCD camera dramatically increase the sampling density and make the scDCT device more portable and less expensive than DCS/DCT. The scDCT system has been tested for 3-D imaging of flow distributions in tissue-simulating phantoms, human forearms, and mastectomy skin flaps (Chong Huang et al, Medical physics, 2015; Chong Huang et al, IEEE Transactions on Medical Imaging, 2017; Siavash Mazdeyasna et al, Journal of Biomedical Optics, 2018). Results demonstrate that scDCT enables a fast and high-density 3-D flow imaging of deep tissue volumes over an adjustable region of interest.
 |
An innovative noncontact speckle contrast diffuse correlation tomography (scDCT) system was downscaled and adapted for noninvasive imaging of cerebral blood flow (CBF) distributions in rat brain through intact scalp and skull. (Chong Huang et al, NeuroImage, 2018).
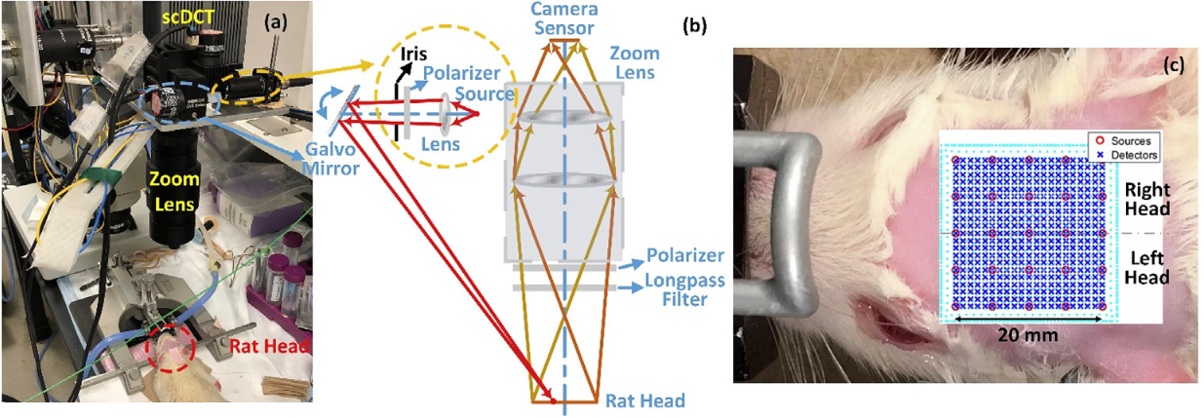
The scDCT instrument was moved to the surgical room for intraoperative 3-D imaging of blood flow distribution in a mastectomy skin flap. The expected spatial heterogeneities of blood flow distributions in the measured tissue volumes for the patients were observed (Siavash Mazdeyasna et al, Journal of Biomed Optics, 2018).
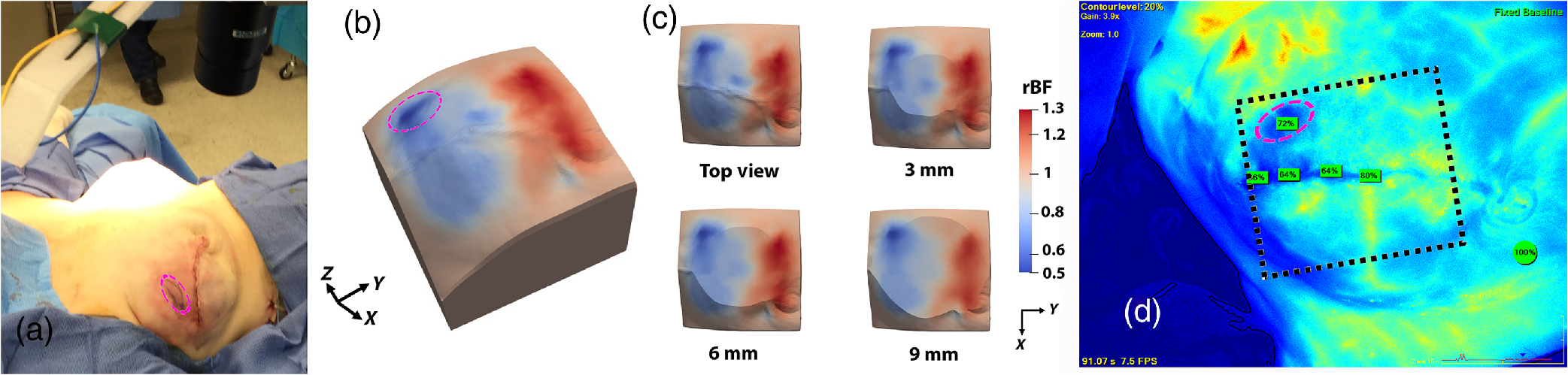 |
We adapted the novel scDCT for noncontact 3D imaging of blood flow (BF) distributions in burn wounds of two patients as a proof of concept. Knowledge of BF distributions in injured tissues is essential for improving acute management of battlefield injuries in combat surgical hospitals (Mingjun Zhao et al, Military Medicine, 2020).
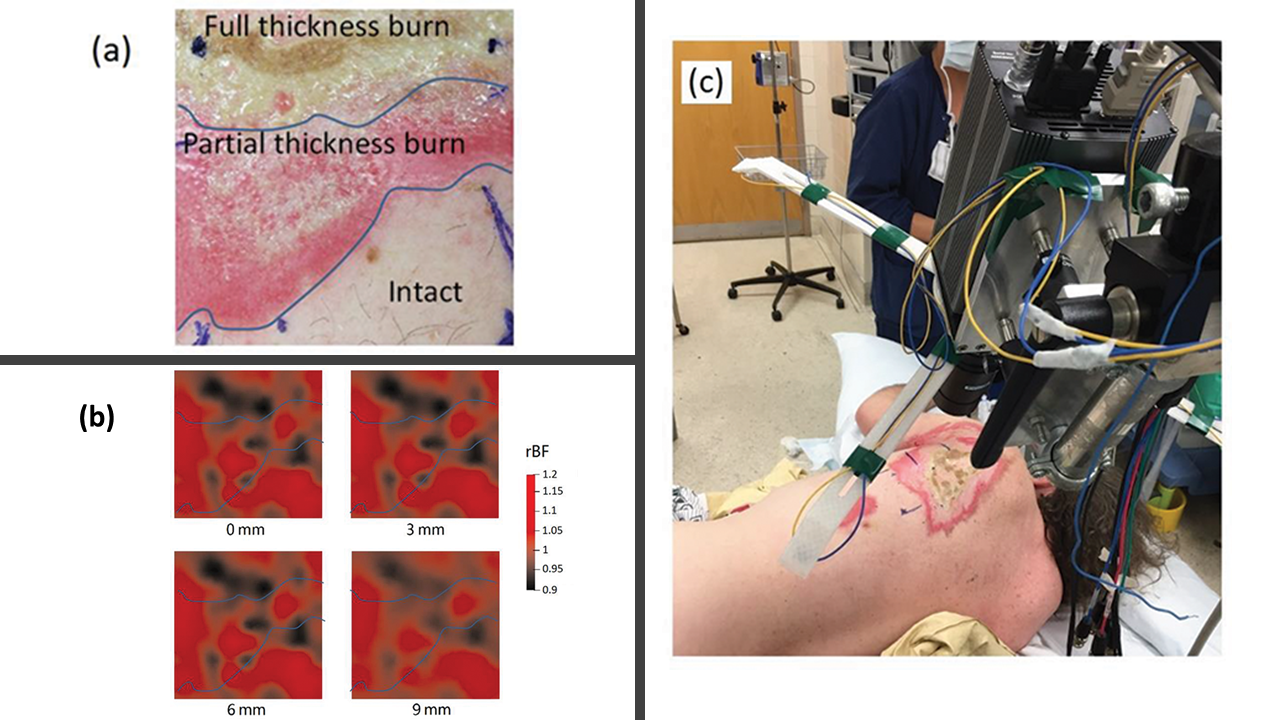
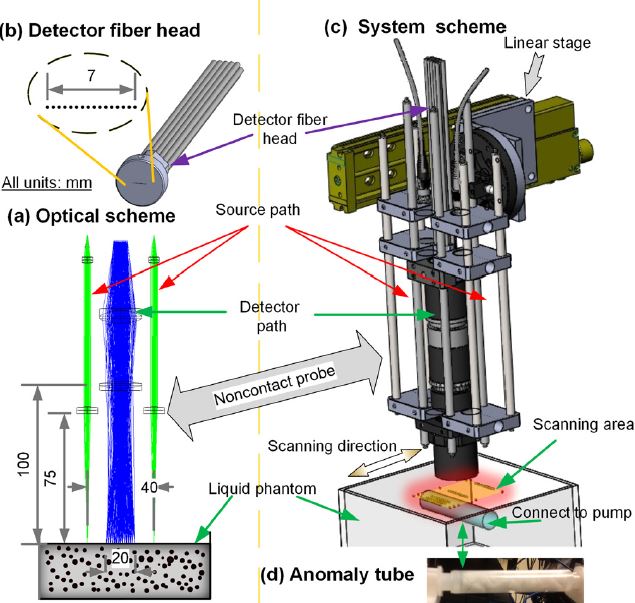
Noncontact diffuse correlation tomography (ncDCT). This study extended our noncontact DCS system into ncDCT for 3-D flow imaging of deep tissue. A linear array of 15 photodetectors and two laser sources connected to a mobile lens-focusing system enabled automatic and noncontact scanning of flow in a region of interest. These boundary measurements were combined with a novel finite element framework for ncDCT image reconstruction implemented into an existing software package (NIRFAST). This new technique was tested in computer simulations and using an innovative tissue-like phantom with anomaly flow contrast design (Yu Lin et al, Applied Physics Letters, 2014). The results exhibit promise of our unique ncDCT technique for 3-D imaging of deep tissue blood flow heterogeneities. Very recently, we have tested this noncontact DCT system for imaging of mammary tumors in mice and human breasts.
Diffuse speckle contrast flow-oximetry (DSCFO; US patent: US2018/0020962). This study focuses on a wearable, fiber-free, fast, and low-cost near-infrared optical sensor which enables noninvasive measurements of cerebral blood flow and oxygenation. The DSCFO uses small laser diodes as focused point sources for deep tissue penetration and a tiny CMOS camera as a high-density 2D detector array to detect spontaneous spatial fluctuations of diffuse laser speckles, resulting from movement of red blood cells in the deep brain (i.e., CBF) (Chong Huang et al, IEEE J Sel Top Quantum Electron, 2019; Chong Huang et al, Journal of Biomedical Optics, 2016). Importantly, connections between the DSCFO probe and a control device are all flexible electrical wires (i.e., fiber-free), providing the promise for continuous cerebral monitoring in freely behaving subjects.
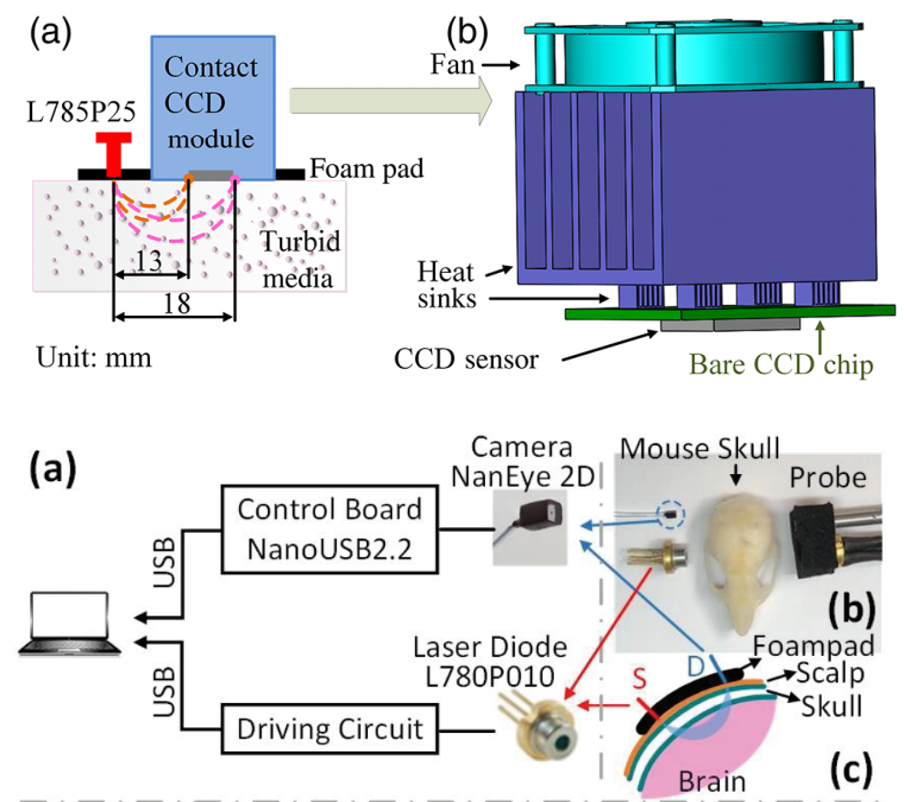 |
|
Dual-wavelength Diffuse Correlation Spectroscopy (DCS) Tissue Flow-Oximeter. We have combined the DCS with a commercial near-infrared spectroscopy (Imagent, ISS, IL) to form a hybrid optical instrument for simultaneous measurements of tissue blood flow and oxygenation. However, the hybrid instruments are still relatively large, complex and expensive. Thus we sought to build and validate a portable, easy-to-use, and inexpensive optical device for bedside monitoring of deep tissue hemodynamics. For this purpose, we added a second laser diode to a portable DCS flowmeter and measured light intensities at two wavelengths (785 and 854 nm) to extract tissue oxygenation information. We name this device the “DCS flow-oximeter” as it measures both blood flow and oxygenation (Yu Shang et al, Optics Letters, 2009). More than 10 peer-reviewed papers have been published using this portable device for the monitoring of tissue blood flow and oxygenation changes in brain, muscle, and tumor.
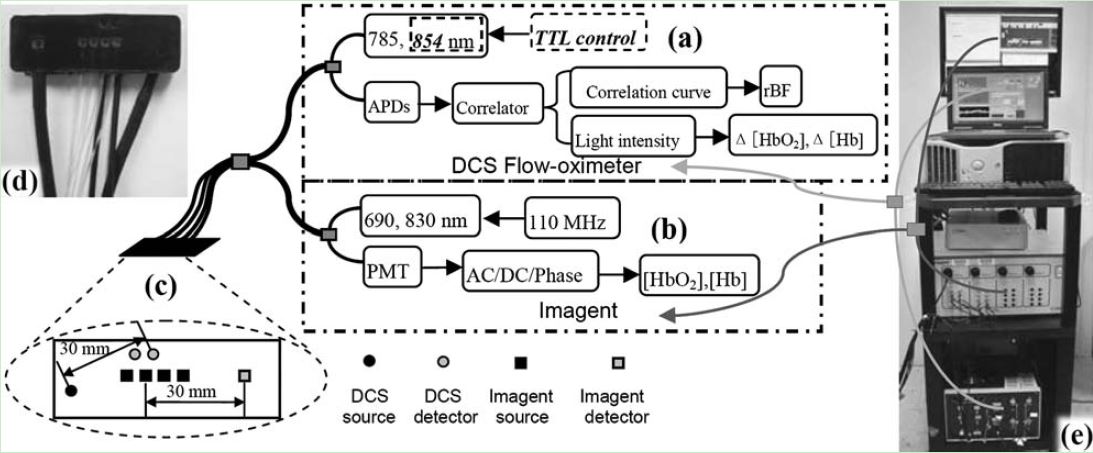
We have used the hybrid instrument (Imagent + DCS) to noninvasively and simultaneously detect low frequency oscillations (LFOs) around 0.1 Hz of cerebral blood flow (CBF) and cerebral oxygenation in human prefrontal cortex (Ran Cheng et al, NeuroImage, 2012, Ran Cheng at al, Journal of Biomedical Optics, 2014). We also tested the feasibility and sensitivity of the dual-wavelength DCS flow-oximeter in patients undergoing carotid endarterectomy (Yu Shang et al, Physics in Medicine and Biology, 2011), in mice with stroke (Yu Shang et al, Optics Express, 2011; Lei Chen et al, Journal of Experimental Stroke and Translational Medicine, 2012).
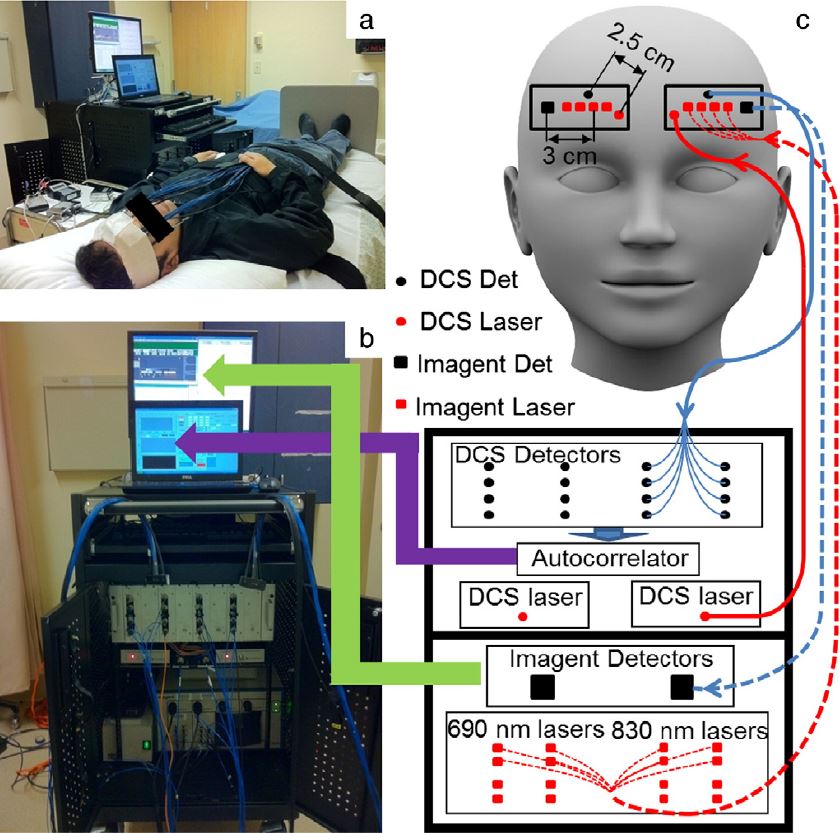
Noncontact DCS flow-oximeter. Significant problems with contact measurements include the risk for infection of vulnerable tissues and the deformation of soft tissues (e.g., breast) distorting tissue hemodynamics. Only a few studies have used noncontact DCS probes to monitor blood flow in murine tumors or rat brains. In these studies, the source and detector fibers were projected on the tissue surface by a camera lenses aligned in a single optical path for both light delivery and detection. The shared optical path limited the maximum source-detector (S-D) separations (<10 mm). Recently my group designed a unique noncontact probe with two separated/isolated optical paths for source and detector respectively (Yu Lin et al, Journal of Biomedical Optics Letters, 2012; T. Li et al, Scientific Reports, 2013). This unique design allowed for setting large S-D separations as needed (e.g., ≥ 25 mm). To the best of our knowledge, this is the first successful noncontact DCS flow-oximeter system for probing blood flow and oxygenation in deep tissues, which holds potential for measuring both blood flow and oxygenation in vulnerable (e.g., pressure ulcer) and soft (e.g., breast) tissues without distorting tissue hemodynamic properties.
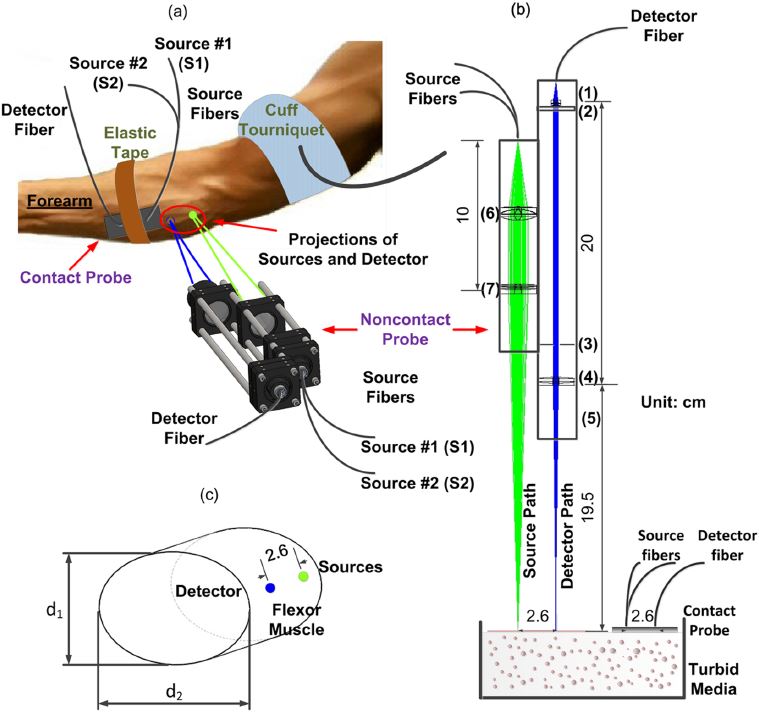
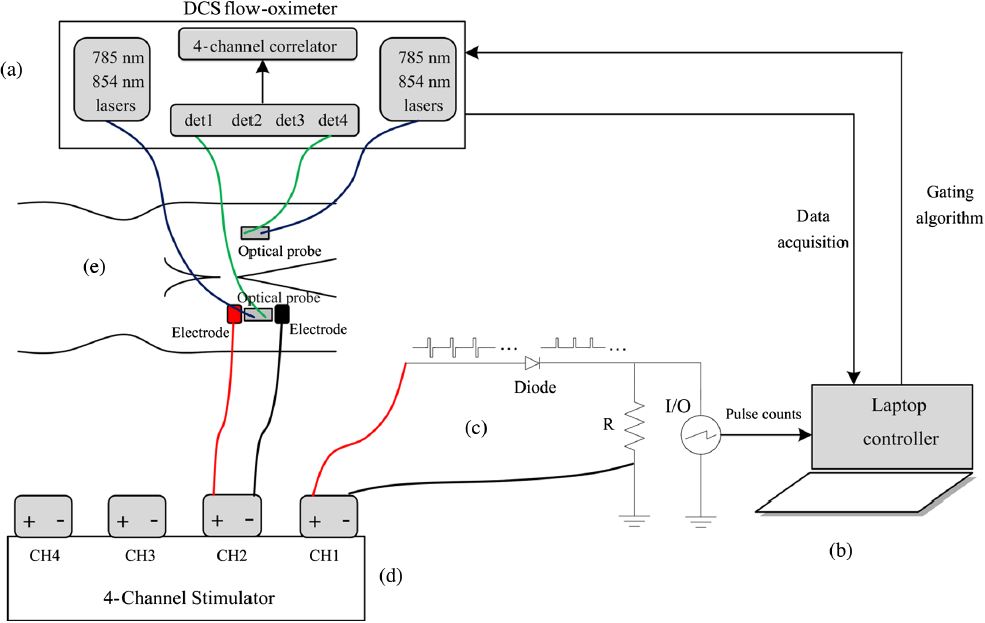
Occlusion protocol and gating algorithm for absolute and continuous measurements of muscle hemodynamic responses during exercise. This study investigates a method using hybrid diffuse optical spectroscopies (Imagent and DCS) to obtain continuous, noninvasive measurement of absolute blood flow (BF), blood oxygenation, and oxygen consumption rate () in exercising skeletal muscle. Healthy subjects performed handgrip exercise to increase BF and in forearm flexor muscles, while a hybrid optical probe on the skin surface directly monitored oxy-, deoxy-, and total hemoglobin concentrations ([HbO2], [Hb], and THC), tissue oxygen saturation (StO2), relative blood flow (rBF), and relative oxygen consumption rate (r). The rBF and r signals were calibrated with absolute baseline BF and obtained through venous and arterial occlusions, respectively. Known problems with muscle fiber motion artifact in optical measurements during exercise were mitigated using a novel gating algorithm determining muscle contraction status based on control signals from a dynamometer. Results were consistent with previous literature findings (Katelyn Gurley et al, Journal of Biomedical Optics, 2012). The protocol/algorithm was also translated from arm to leg to monitor muscle responses to exercises or electrical stimulations (Yu Shang et al, Journal of Biomedical Optics, 2013). Our studies support the application of NIRS/DCS technology to quantitatively evaluate hemodynamic and metabolic parameters in exercising/stimulating skeletal muscles, which holds promising potential for improving diagnosis and treatment evaluation for patients suffering from diseases affecting skeletal muscle.